Search
- Page Path
- HOME > Search
- Thyroid
- Active Surveillance for Low-Risk Papillary Thyroid Carcinoma as an Acceptable Management Option with Additional Benefits: A Comprehensive Systematic Review
- Jee Hee Yoon, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2024;39(1):152-163. Published online January 22, 2024
- DOI: https://doi.org/10.3803/EnM.2023.1794
- 1,161 View
- 42 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
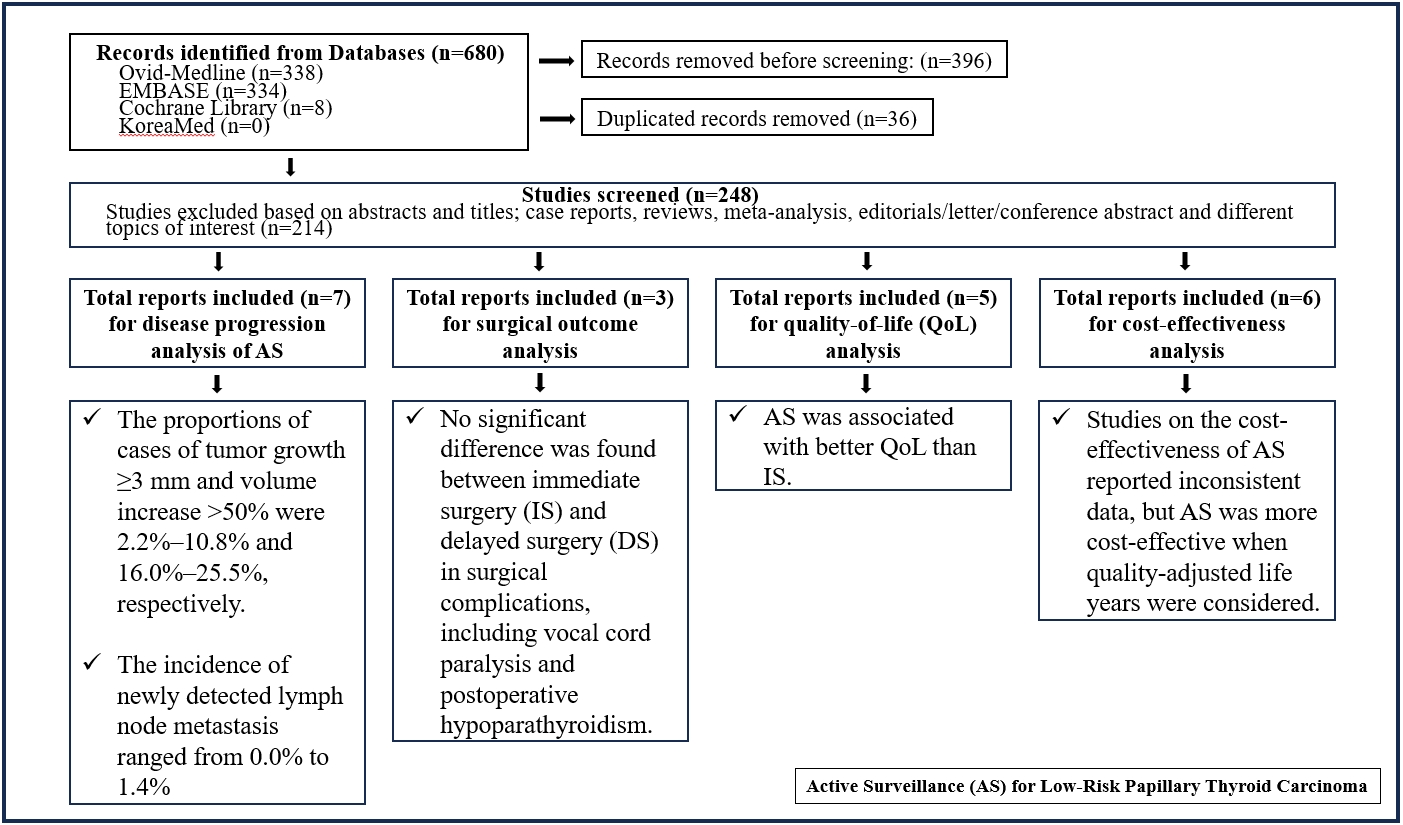
Active surveillance (AS) has been introduced as a management strategy for low-risk papillary thyroid carcinoma (PTC) due to its typically indolent nature. Despite this, the widespread adoption of AS has encountered several challenges. The aim of this systematic review was to evaluate the safety of AS related to disease progression and its benefits compared with immediate surgery (IS).
Methods
Studies related to AS in patients with low-risk PTC were searched through the Ovid MEDLINE, Embase, Cochrane Library, and KoreaMed databases. Studies on disease progression, surgical complication, quality of life (QoL), and cost-effectiveness were separately analyzed and narratively synthesized.
Results
In the evaluation of disease progression, the proportions of cases with tumor growth ≥3 mm and a volume increase >50% were 2.2%–10.8% and 16.0%–25.5%, respectively. Newly detected lymph node metastasis was identified in 0.0%–1.4% of patients. No significant difference was found between IS and delayed surgery in surgical complications, including vocal cord paralysis and postoperative hypoparathyroidism. AS was associated with better QoL than IS. Studies on the cost-effectiveness of AS reported inconsistent data, but AS was more cost-effective when quality-adjusted life years were considered.
Conclusion
AS is an acceptable management option for patients with low-risk PTC based on the low rate of disease progression and the absence of an increased mortality risk. AS has additional benefits, including improved QoL and greater QoL-based cost-effectiveness. -
Citations
Citations to this article as recorded by- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma
Kyeong Jin Kim
Endocrinology and Metabolism.2024; 39(1): 95. CrossRef
- It Is Time to Understand the Additional Benefits of Active Surveillance for Low-Risk Papillary Thyroid Carcinoma

- Thyroid
Thyroid Cancer Screening - A Comprehensive Assessment of the Harms of Fine-Needle Aspiration Biopsy for Thyroid Nodules: A Systematic Review
- Ji Yong Park, Wonsuk Choi, A Ram Hong, Jee Hee Yoon, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2023;38(1):104-116. Published online February 27, 2023
- DOI: https://doi.org/10.3803/EnM.2023.1669
- 3,676 View
- 161 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
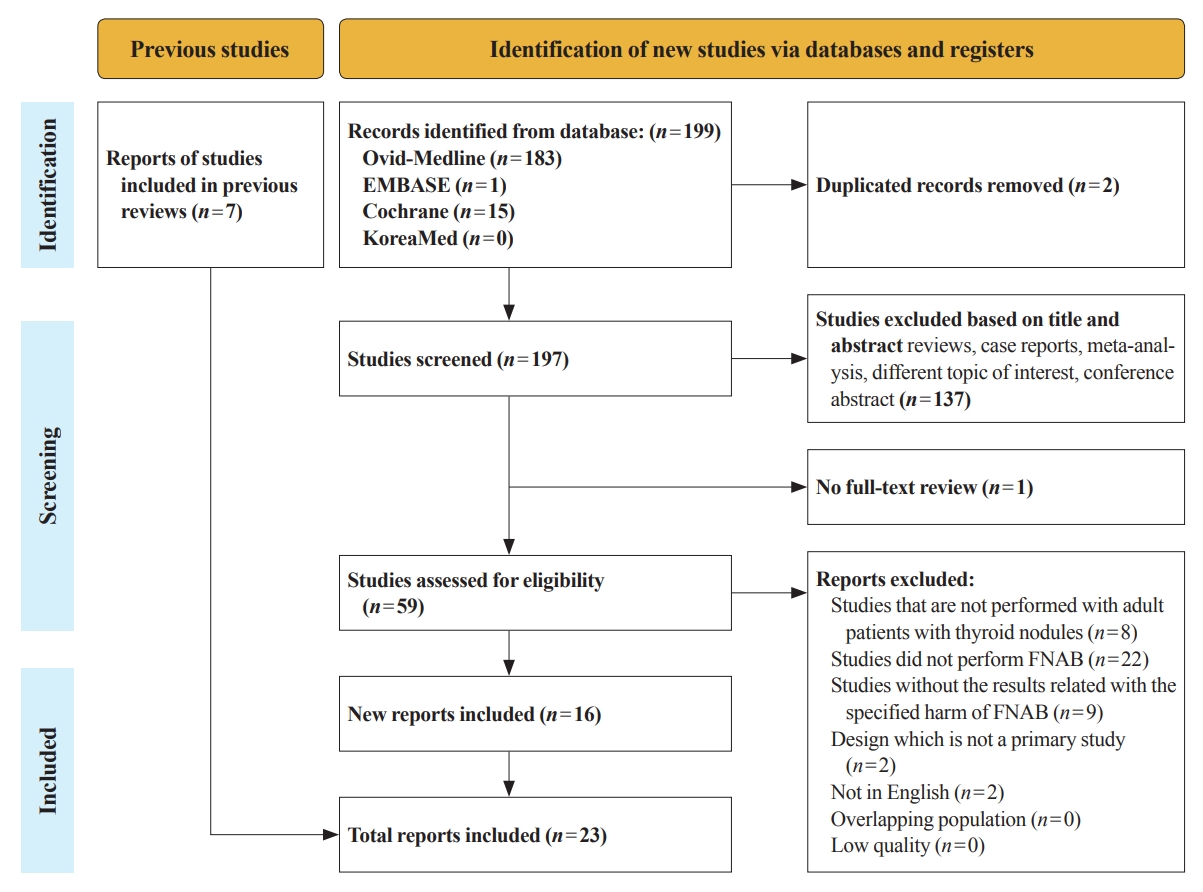
There have concerns related with the potential harms of fine-needle aspiration biopsy (FNAB). We aimed to summarize the clinical complications and evaluate the safety of FNAB.
Methods
Studies related with the harms of FNAB were searched on MEDLINE, Embase, Cochrane library, and KoreaMed from 2012 to 2022. Also, studies reviewed in the previous systematic reviews were evaluated. Included clinical complications were postprocedural pain, bleeding events, neurological symptoms, tracheal puncture, infections, post-FNAB thyrotoxicosis, and needle tract implantation of thyroid cancers.
Results
Twenty-three cohort studies were included in this review. Nine studies which were related with FNAB-related pain showed that most of the subjects had no or mild discomfort. The 0% to 6.4% of the patients had hematoma or hemorrhage after FNAB, according to 15 studies. Vasovagal reaction, vocal cord palsy, and tracheal puncture have rarely described in the included studies. Needle tract implantation of thyroid malignancies was described in three studies reporting 0.02% to 0.19% of the incidence rate.
Conclusion
FNAB is considered to be a safe diagnostic procedure with rare complications, which are mainly minor events. Thorough assessement of the patients’ medical condition when deciding to perform FNABs would be advisable to lower potential complications. -
Citations
Citations to this article as recorded by- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules
Eun Kyung Lee, Young Joo Park, Chan Kwon Jung, Dong Gyu Na
Endocrinology and Metabolism.2024; 39(1): 61. CrossRef - Fine-needle aspiration cytology for neck lesions in patients with antithrombotic/anticoagulant medications: systematic review and meta-analysis
Dongbin Ahn, Ji Hye Kwak, Gill Joon Lee, Jin Ho Sohn
European Radiology.2024;[Epub] CrossRef - To Screen or Not to Screen?
Do Joon Park
Endocrinology and Metabolism.2023; 38(1): 69. CrossRef - Thyroid Cancer Screening: How to Maximize Its Benefits and Minimize Its Harms
Jung Hwan Baek
Endocrinology and Metabolism.2023; 38(1): 75. CrossRef - Evaluation of the Appropriateness of Thyroid Fine-Needle Aspiration
Lairce Cristina Ribeiro Brito, Iara Beatriz De Carvalho Botêlho, Lanna Matos Silva Fernandes, Nayze Lucena Sangreman Aldeman, Uziel Nunes Silva
International Journal for Innovation Education and Research.2023; 11(6): 8. CrossRef
- A Narrative Review of the 2023 Korean Thyroid Association Management Guideline for Patients with Thyroid Nodules

- Thyroid
- Clinical Characteristics and Prognosis of Coexisting Thyroid Cancer in Patients with Graves’ Disease: A Retrospective Multicenter Study
- Jee Hee Yoon, Meihua Jin, Mijin Kim, A Ram Hong, Hee Kyung Kim, Bo Hyun Kim, Won Bae Kim, Young Kee Shong, Min Ji Jeon, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(6):1268-1276. Published online November 26, 2021
- DOI: https://doi.org/10.3803/EnM.2021.1227
- 4,839 View
- 186 Download
- 10 Web of Science
- 11 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
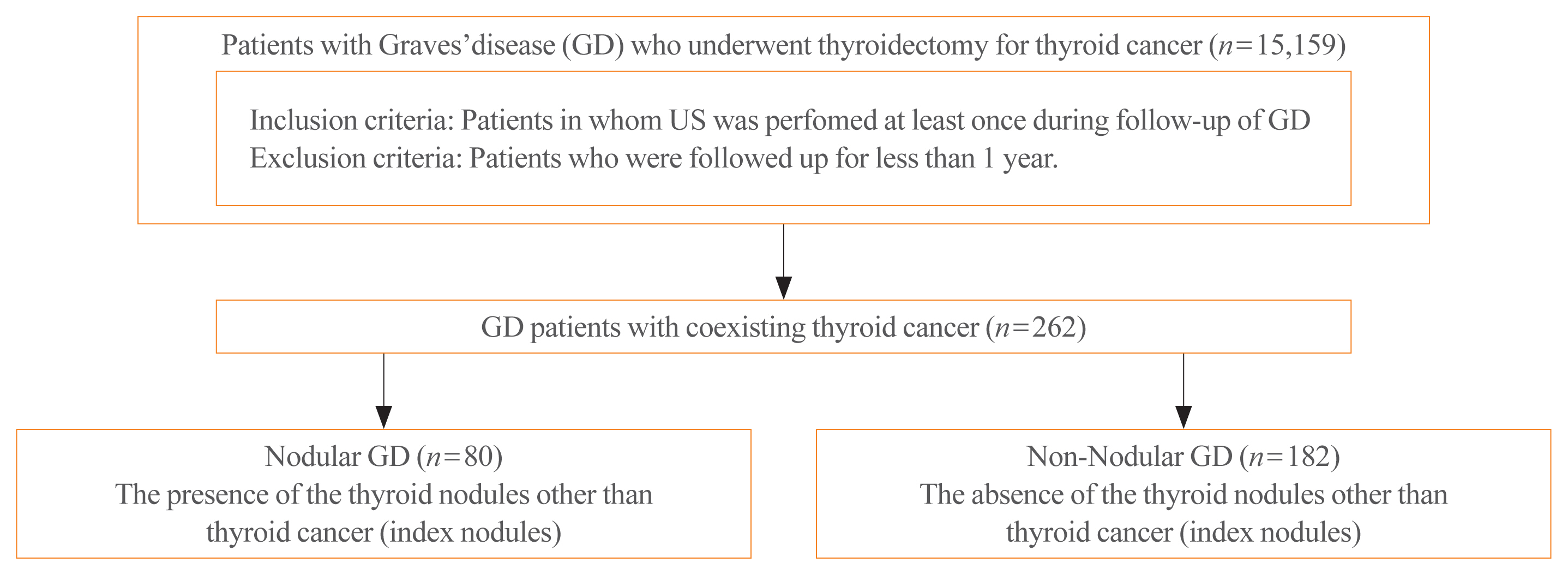
The association between Graves’ disease (GD) and co-existing thyroid cancer is still controversial and most of the previously reported data have been based on surgically treated GD patients. This study investigated the clinicopathological findings and prognosis of concomitant thyroid cancer in GD patients in the era of widespread application of ultrasonography.
Methods
Data of GD patients who underwent thyroidectomy for thyroid cancer between 2010 and 2019 in three tertiary hospitals in South Korea (Asan Medical Center, Chonnam National University Hwasun Hospital, and Pusan National University Hospital) were collected and analyzed retrospectively. In the subgroup analysis, aggressiveness and clinical outcomes of thyroid cancer were compared nodular GD and non-nodular GD groups according to the presence or absence of the thyroid nodules other than thyroid cancer (index nodules).
Results
Of the 15,159 GD patients treated at the hospitals during the study period, 262 (1.7%) underwent thyroidectomy for coexisting thyroid cancer. Eleven patients (4.2%) were diagnosed with occult thyroid cancer and 182 patients (69.5%) had microcarcinomas. No differences in thyroid cancer aggressiveness, ultrasonographic findings, or prognosis were observed between the nodular GD and non-nodular GD groups except the cancer subtype. In the multivariate analysis, only lymph node (LN) metastasis was an independent prognostic factor for recurrent/persistent disease of thyroid cancer arising in GD (P=0.020).
Conclusion
The prevalence of concomitant thyroid cancer in GD patients was considerably lower than in previous reports. The clinical outcomes of thyroid cancer in GD patients were also excellent but, more cautious follow-up is necessary for patients with LN metastasis in the same way as for thyroid cancer in non-GD patients. -
Citations
Citations to this article as recorded by- Comparison of Surgical Outcomes of Transoral Versus Open Thyroidectomy for Graves Disease
Suo-Hsien Wang, Wu-Po Chao, Ta-You Lo, Soh-Ching Ng, Yu-Hsien Chen
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2024; 34(2): 150. CrossRef - Outcomes of Surgical Treatment for Graves’ Disease: A Single-Center Experience of 216 Cases
Hanxing Sun, Hui Tong, Xiaohui Shen, Haoji Gao, Jie Kuang, Xi Chen, Qinyu Li, Weihua Qiu, Zhuoran Liu, Jiqi Yan
Journal of Clinical Medicine.2023; 12(4): 1308. CrossRef - Cancer and Mortality Risks of Graves’ Disease in South Korea Based on National Data from 2010 to 2019
Young Ju Choi, Kyungdo Han, Won Kyoung Cho, Min Ho Jung, Byung-Kyu Suh
Clinical Epidemiology.2023; Volume 15: 535. CrossRef - Risk and Prognosis of Thyroid Cancer in Patients with Graves’ Disease: An Umbrella Review
Marco Palella, Francesca Maria Giustolisi, Adriana Modica Fiascaro, Martina Fichera, Antonella Palmieri, Rossella Cannarella, Aldo E. Calogero, Margherita Ferrante, Maria Fiore
Cancers.2023; 15(10): 2724. CrossRef - Characteristics, staging and outcomes of differentiated thyroid cancer in patients with and without Graves’ disease
Chaitra Gopinath, Hanna Crow, Sujata Panthi, Leonidas Bantis, Kenneth D. Burman, Chitra Choudhary
Journal of Clinical & Translational Endocrinology.2023; 33: 100321. CrossRef - Prevalence, Treatment Status, and Comorbidities of Hyperthyroidism in Korea from 2003 to 2018: A Nationwide Population Study
Hwa Young Ahn, Sun Wook Cho, Mi Young Lee, Young Joo Park, Bon Seok Koo, Hang-Seok Chang, Ka Hee Yi
Endocrinology and Metabolism.2023; 38(4): 436. CrossRef - Hashimoto’s Thyroiditis and Papillary Thyroid Carcinoma: A Follow-Up Study in Patients with Absence of Aggressive Risk Factors at the Surgery of the Primary Tumor
Andrea Marongiu, Susanna Nuvoli, Andrea De Vito, Sonia Vargiu, Angela Spanu, Giuseppe Madeddu
Diagnostics.2023; 13(19): 3068. CrossRef - Table of Contents
Clinical Thyroidology.2022; 34(2): 48. CrossRef - Predisposition to and Prognosis of Thyroid Cancer May Not Be Affected by Graves’ Disease, But Some Questions Still Remain
Yanrui Huang, Haixia Guan
Clinical Thyroidology.2022; 34(2): 59. CrossRef - A Comparative Follow-Up Study of Patients with Papillary Thyroid Carcinoma Associated or Not with Graves’ Disease
Andrea Marongiu, Susanna Nuvoli, Andrea De Vito, Maria Rondini, Angela Spanu, Giuseppe Madeddu
Diagnostics.2022; 12(11): 2801. CrossRef - An unusual case of papillary thyroid carcinoma presenting as Graves’ disease
Pooja Tiwari, Uma Kaimal Saikia, Abhamoni Baro, Ashok Krishna Bhuyan
Thyroid Research and Practice.2022; 19(1): 47. CrossRef
- Comparison of Surgical Outcomes of Transoral Versus Open Thyroidectomy for Graves Disease

- Thyroid
- Programmed Cell Death-Ligand 1 (PD-L1) gene Single Nucleotide Polymorphism in Graves’ Disease and Hashimoto’s Thyroiditis in Korean Patients
- Jee Hee Yoon, Min-ho Shin, Hee Nam Kim, Wonsuk Choi, Ji Yong Park, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(3):599-606. Published online June 2, 2021
- DOI: https://doi.org/10.3803/EnM.2021.965
- 3,999 View
- 117 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Programmed cell death-ligand 1 (PD-L1) has an important role in regulating immune reactions by binding to programmed death 1 (PD-1) on immune cells, which could prevent the exacerbation of autoimmune thyroid disease (AITD). The aim of this study was to evaluate the association of PD-L1 polymorphism with AITD, including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT).
Methods
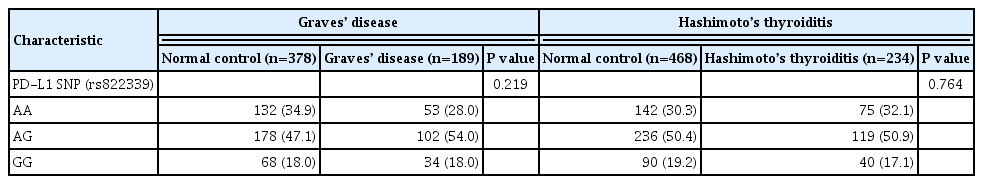
A total of 189 GD patients, 234 HT patients, and 846 healthy age- and sex-matched controls were enrolled in this study. We analyzed PD-L1 single nucleotide polymorphism (SNP) (rs822339) and investigated the associations with clinical disease course and outcome.
Results
Genotype frequency at the PD-L1 marker RS822339 in GD (P=0.219) and HT (P=0.764) patients did not differ from that among healthy controls. In patients with GD, the A/G or G/G genotype group demonstrated higher TBII titer (20.6±20.5 vs. 28.0± 25.8, P=0.044) and longer treatment duration (39.0±40.4 months vs. 62.4±65.0 months, P=0.003) compared to the A/A genotype group. Among patients in whom anti-thyroid peroxidase (TPO) antibody was measured after treatment of GD, post-treatment antiTPO positivity was higher in the A/G or G/G genotype group compared to the A/A genotype group (48.1% vs. 69.9%, P=0.045). Among patients with HT, there was no significant difference of anti-TPO antibody positivity (79.4% vs. 68.6%, P=0.121), anti-thyroglobulin antibody positivity (80.9% vs. 84.7%, P=0.661), or development to overt hypothyroidism (68.0% vs. 71.1%, P=0.632) between the A/A genotype group and the A/G or G/G genotype group.
Conclusion
The genotype frequency of PD-L1 (rs822339) is not different in patients with AITD compared with healthy controls. The intact PD-1/PD-L1 pathway in GD and HT might be important to maintain chronicity of AITD by protecting immune tolerance. However, the PD-L1 SNP could be associated with difficulty in achieving remission in patients with GD, which may be helpful to predict the possibility of longer treatment. Further studies are required to investigate the complex immune tolerance system in patients with AITD. -
Citations
Citations to this article as recorded by- Synergistic effects of BTN3A1, SHP2, CD274, and STAT3 gene polymorphisms on the risk of systemic lupus erythematosus: a multifactorial dimensional reduction analysis
Yang-Yang Tang, Wang-Dong Xu, Lu Fu, Xiao-Yan Liu, An-Fang Huang
Clinical Rheumatology.2024; 43(1): 489. CrossRef - Relationship between CD274 gene polymorphism and systemic lupus erythematosus risk in a Chinese Han population
Lu‐Qi Yang, Zhen Qin, Lu Fu, Wang‐Dong Xu
International Journal of Rheumatic Diseases.2024;[Epub] CrossRef
- Synergistic effects of BTN3A1, SHP2, CD274, and STAT3 gene polymorphisms on the risk of systemic lupus erythematosus: a multifactorial dimensional reduction analysis

- Clinical Study
- Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors
- Jee Hee Yoon, A Ram Hong, Hee Kyung Kim, Ho-Cheol Kang
- Endocrinol Metab. 2021;36(2):413-423. Published online April 6, 2021
- DOI: https://doi.org/10.3803/EnM.2020.906

- 5,648 View
- 218 Download
- 23 Web of Science
- 22 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
Thyroid immune-related adverse events (IRAEs) have been reported in patients treated with programmed cell death protein-1 (PD-1) and programmed cell death protein-ligand 1 (PD-L1) inhibitors. We investigated the incidence and clinical course of PD-1/PD-L1 inhibitor-induced thyroid IRAEs, and identified predictable clinical risk factors of thyroid IRAEs, in particular, overt hypothyroidism (OH).
Methods
We retrospectively reviewed the medical records of 325 cancer patients receiving PD-1/PD-L1 inhibitor in a tertiary referral center.
Results
A total of 50.5% (164/325) of patients experienced at least one abnormal thyroid function following PD-1/PD-L1 inhibitor. Eighty-four patients (51.2%) of them recovered to normal thyroid function during follow-up. In overall population, 25 patients (7.7%) required thyroid hormone replacement therapy due to PD-1/PD-L1 inhibitor-induced OH. Patients who progressed to OH showed significantly higher baseline thyroid stimulating hormone level and longer duration of PD-1/PD-L1 inhibitor therapy than those without thyroid dysfunction or OH (both P<0.001). Median time interval to the development of OH was 3 months after the therapy. OH was significantly associated with positive anti-thyroid peroxidase antibody at baseline and anti-thyroglobulin antibody during the therapy than those without thyroid dysfunction or OH (P=0.015 and P=0.005, respectively). We observed no patients with OH who were able to stop levothyroxine replacement after the cessation of PD-1/PD-L1 inhibitor therapy.
Conclusion
PD-1/PD-L1 inhibitor-induced thyroid dysfunctions are considerably reversible; however, OH is irreversible requiring levothyroxine replacement even after stopping the therapy. Positive thyroid autoantibodies may predict the progression to OH. -
Citations
Citations to this article as recorded by- Thyroid Dysfunction after Atezolizumab and Bevacizumab Is Associated with Favorable Outcomes in Hepatocellular Carcinoma
Young Shin Song, Hannah Yang, Beodeul Kang, Jaekyung Cheon, Ilhwan Kim, Hyeyeong Kim, Won Suk Lee, Yun Beom Sang, Sanghoon Jung, Ho Yeong Lim, Vincent E. Gaillard, Chan Kim, Hong Jae Chon
Liver Cancer.2024; 13(1): 89. CrossRef - Thyroid dysfunction (TD) induced by PD-1/PD-L1 inhibitors in advanced lung cancer
Yanling Wang, Xiaoxuan Yang, Jia Ma, Shenglan Chen, Ping Gong, Ping Dai
Heliyon.2024; 10(5): e27077. CrossRef - Non-Invasive Predictive Biomarkers for Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors
Ben Ponvilawan, Abdul Wali Khan, Janakiraman Subramanian, Dhruv Bansal
Cancers.2024; 16(6): 1225. CrossRef - Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy
David Dora, Syeda Mahak Zahra Bokhari, Kenan Aloss, Peter Takacs, Juliane Zsuzsanna Desnoix, György Szklenárik, Patrick Deniz Hurley, Zoltan Lohinai
International Journal of Molecular Sciences.2023; 24(3): 2769. CrossRef - Immune checkpoint inhibitor-associated toxicity in advanced non-small cell lung cancer: An updated understanding of risk factors
Xiangxiao Hu, Lina Wang, Bin Shang, Junren Wang, Jian Sun, Bin Liang, Lili Su, Wenjie You, Shujuan Jiang
Frontiers in Immunology.2023;[Epub] CrossRef - Immune-related adverse events induced by programmed death protein-1 inhibitors from the perspective of lymphoma immunotherapy
Yong-Zhe Hou, Qin Zhang, Hai Bai, Tao Wu, Ya-Jie Chen
World Journal of Clinical Cases.2023; 11(7): 1458. CrossRef - Predictive Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Events
Iñigo Les, Mireia Martínez, Inés Pérez-Francisco, María Cabero, Lucía Teijeira, Virginia Arrazubi, Nuria Torrego, Ana Campillo-Calatayud, Iñaki Elejalde, Grazyna Kochan, David Escors
Cancers.2023; 15(5): 1629. CrossRef - Immune-related thyroid dysfunctions during anti PD-1/PD-L1 inhibitors: new evidence from a single centre experience
Alice Nervo, Matteo Ferrari, Giovanni Gruosso, Enrica Migliore, Sara Basile, Valentina D’Angelo, Anna Roux, Alessandro Piovesan, Emanuela Arvat
Clinical and Experimental Medicine.2023; 23(8): 4817. CrossRef - RNA Sequencing Reveals Unique Transcriptomic Signatures of the Thyroid in a Murine Lung Cancer Model Treated with PD-1 and PD-L1 Antibodies
Rena Pollack, Joshua Stokar, Natan Lishinsky, Irina Gurt, Naomi Kaisar-Iluz, Merav E. Shaul, Zvi G. Fridlender, Rivka Dresner-Pollak
International Journal of Molecular Sciences.2023; 24(13): 10526. CrossRef - Immune‐related adverse events after immune check point inhibitors: Understanding the intersection with autoimmunity
Namrata Singh, Anne M. Hocking, Jane H. Buckner
Immunological Reviews.2023; 318(1): 81. CrossRef - Endocrine Side Effects in Patients Treated with Immune Checkpoint Inhibitors: A Narrative Review
Nicia I. Profili, Roberto Castelli, Antonio Gidaro, Alessandro Merella, Roberto Manetti, Giuseppe Palmieri, Margherita Maioli, Alessandro P. Delitala
Journal of Clinical Medicine.2023; 12(15): 5161. CrossRef - Incidence of Endocrine-Related Dysfunction in Patients Treated with New Immune Checkpoint Inhibitors: A Meta-Analysis and Comprehensive Review
Won Sang Yoo, Eu Jeong Ku, Eun Kyung Lee, Hwa Young Ahn
Endocrinology and Metabolism.2023; 38(6): 750. CrossRef - PD-1/PD-L1 Inhibitors in Patients With Preexisting Autoimmune Diseases
Ke Zhang, Xiangyi Kong, Yuan Li, Zhongzhao Wang, Lin Zhang, Lixue Xuan
Frontiers in Pharmacology.2022;[Epub] CrossRef - Association between the type of thyroid dysfunction induced by immune checkpoint inhibitors and prognosis in cancer patients
Han-sang Baek, Chaiho Jeong, Kabsoo Shin, Jaejun Lee, Heysun Suh, Dong-Jun Lim, Moo Il Kang, Jeonghoon Ha
BMC Endocrine Disorders.2022;[Epub] CrossRef - A Successful Case of Hepatocellular Carcinoma Treated with Atezolizumab Plus Bevacizumab with Multisystem Immune-related Adverse Events
Hidemi Hayashi, Koji Sawada, Takumu Hasebe, Shunsuke Nakajima, Jun Sawada, Yuri Takiyama, Yumi Takiyama, Toshikatsu Okumura, Mikihiro Fujiya
Internal Medicine.2022; 61(23): 3497. CrossRef - Immune Related Adverse Events of the Thyroid – A Narrative Review
Christopher A. Muir, Venessa H. M. Tsang, Alexander M. Menzies, Roderick J. Clifton-Bligh
Frontiers in Endocrinology.2022;[Epub] CrossRef - Thyroid-related adverse events induced by immune checkpoint inhibitors
Alexandra Chera, Andreea Lucia Stancu, Octavian Bucur
Frontiers in Endocrinology.2022;[Epub] CrossRef - Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors
Adithya Chennamadhavuni, Laith Abushahin, Ning Jin, Carolyn J. Presley, Ashish Manne
Frontiers in Immunology.2022;[Epub] CrossRef - Case 5: A 41-Year-Old Woman With Palpitation
Jiwon Yang, Kabsoo Shin, Jeongmin Lee, Jeonghoon Ha, Dong-Jun Lim, Han-Sang Baek
Journal of Korean Medical Science.2022;[Epub] CrossRef - Immune Checkpoint Inhibitors and Endocrine Disorders: A Position Statement from the Korean Endocrine Society
Hyemi Kwon, Eun Roh, Chang Ho Ahn, Hee Kyung Kim, Cheol Ryong Ku, Kyong Yeun Jung, Ju Hee Lee, Eun Heui Kim, Sunghwan Suh, Sangmo Hong, Jeonghoon Ha, Jun Sung Moon, Jin Hwa Kim, Mi-kyung Kim
Endocrinology and Metabolism.2022; 37(6): 839. CrossRef - Management of Endocrine and Metabolic Toxicities of Immune-Checkpoint Inhibitors: From Clinical Studies to a Real-Life Scenario
Calogera Claudia Spagnolo, Giuseppe Giuffrida, Salvatore Cannavò, Tindara Franchina, Nicola Silvestris, Rosaria Maddalena Ruggeri, Mariacarmela Santarpia
Cancers.2022; 15(1): 246. CrossRef - Antineoplastics
Reactions Weekly.2021; 1874(1): 31. CrossRef
- Thyroid Dysfunction after Atezolizumab and Bevacizumab Is Associated with Favorable Outcomes in Hepatocellular Carcinoma

- Clinical Study
- Vandetanib for the Management of Advanced Medullary Thyroid Cancer: A Real-World Multicenter Experience
- Mijin Kim, Jee Hee Yoon, Jonghwa Ahn, Min Ji Jeon, Hee Kyung Kim, Dong Jun Lim, Ho-Cheol Kang, In Joo Kim, Young Kee Shong, Tae Yong Kim, Bo Hyun Kim
- Endocrinol Metab. 2020;35(3):587-594. Published online September 22, 2020
- DOI: https://doi.org/10.3803/EnM.2020.687
- 5,635 View
- 146 Download
- 12 Web of Science
- 12 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
Vandetanib is the most widely used tyrosine kinase inhibitor for the treatment of patients with advanced medullary thyroid cancer (MTC). However, only limited data regarding its use outside clinical trials are available. We aimed to evaluate the efficacy and safety of vandetanib in patients with advanced MTC in routine clinical practice.
Methods
In this multicenter retrospective study, 12 patients with locally advanced or metastatic MTC treated with vandetanib at four tertiary hospitals were included. The primary outcome was the objective response rate (ORR) based on the Response Evaluation Criteria in Solid Tumors. The progression-free survival (PFS), overall survival (OS), and toxicities were also evaluated.
Results
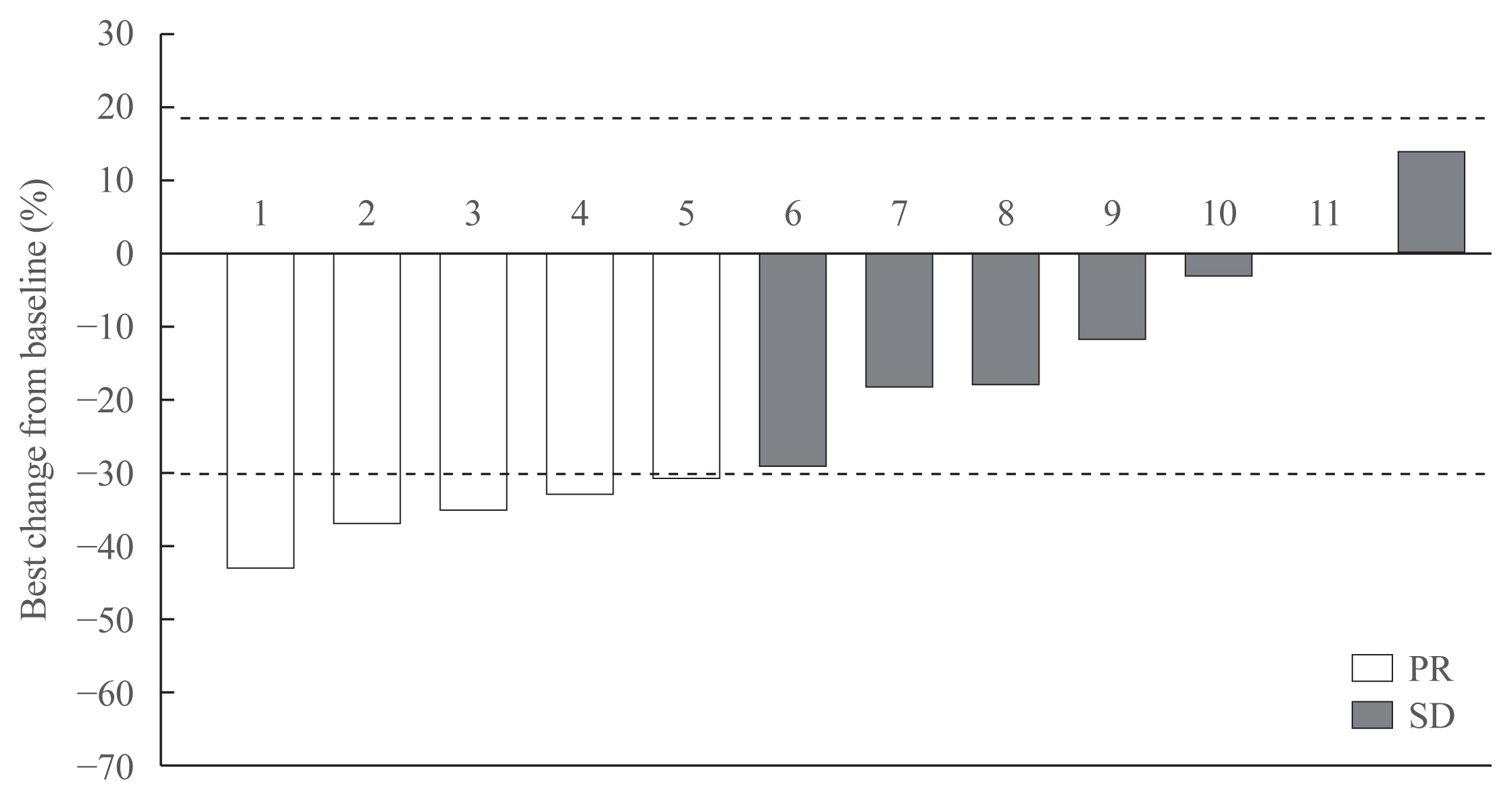
Eleven patients (92%) had distant metastasis and 10 (83%) had disease progression at enrollment. Partial response was observed in five patients (ORR, 42%) and stable disease lasting ≥24 weeks was reported in an additional five patients (83%). During the median 31.7 months of follow-up, disease progression was seen in five patients (42%); of these, two died due to disease progression. The median PFS was 25.9 months, while the median OS was not reached. All patients experienced adverse events (AEs) which were generally consistent with the known safety profile of vandetanib. Vandetanib was discontinued in two patients due to skin toxicity.
Conclusion
Consistent with the phase III trial, this study confirmed the efficacy of vandetanib for advanced MTC in terms of both ORR and PFS in the real-world setting. Vandetanib was well tolerated in the majority of patients, and there were no fatal AEs. -
Citations
Citations to this article as recorded by- Metastatic medullary thyroid carcinoma (MTC): disease course, treatment modalities and factors predisposing for drug resistance
Katerina Saltiki, George Simeakis, Olga Karapanou, Stavroula A. Paschou, Maria Alevizaki
Endocrine.2023; 80(3): 570. CrossRef - Initial Experiences of Selective RET Inhibitor Selpercatinib in Adults with Metastatic Differentiated Thyroid Carcinoma and Medullary Thyroid Carcinoma: Real-World Case Series in Korea
Han-Sang Baek, Jeonghoon Ha, Seunggyun Ha, Ja Seong Bae, Chan Kwon Jung, Dong-Jun Lim
Current Oncology.2023; 30(3): 3020. CrossRef - Molecular Basis and Natural History of Medullary Thyroid Cancer: It is (Almost) All in the RET
Nicolas Sahakian, Frédéric Castinetti, Pauline Romanet
Cancers.2023; 15(19): 4865. CrossRef - Sporadic Medullary Thyroid Carcinoma: Towards a Precision Medicine
Antonio Matrone, Carla Gambale, Alessandro Prete, Rossella Elisei
Frontiers in Endocrinology.2022;[Epub] CrossRef - Targeted therapy and drug resistance in thyroid cancer
Yujie Zhang, Zhichao Xing, Tianyou Liu, Minghai Tang, Li Mi, Jingqiang Zhu, Wenshuang Wu, Tao Wei
European Journal of Medicinal Chemistry.2022; 238: 114500. CrossRef - Daily Management of Patients on Multikinase Inhibitors’ Treatment
Carla Colombo, Simone De Leo, Matteo Trevisan, Noemi Giancola, Anna Scaltrito, Laura Fugazzola
Frontiers in Oncology.2022;[Epub] CrossRef - The Angiogenic Balance and Its Implications in Cancer and Cardiovascular Diseases: An Overview
Cătălina Ionescu, Bogdan Oprea, Georgeta Ciobanu, Milena Georgescu, Ramona Bică, Garofiţa-Olivia Mateescu, Fidan Huseynova, Veronique Barragan-Montero
Medicina.2022; 58(7): 903. CrossRef - Reassessing vascular endothelial growth factor (VEGF) in anti-angiogenic cancer therapy
Tobiloba C. Elebiyo, Damilare Rotimi, Ikponmwosa O. Evbuomwan, Rotdelmwa Filibus Maimako, Matthew Iyobhebhe, Oluwafemi Adeleke Ojo, Olarewaju M. Oluba, Oluyomi S. Adeyemi
Cancer Treatment and Research Communications.2022; 32: 100620. CrossRef - Current Guidelines for Management of Medullary Thyroid Carcinoma
Mijin Kim, Bo Hyun Kim
Endocrinology and Metabolism.2021; 36(3): 514. CrossRef - Recent advances in precision medicine for the treatment of medullary thyroid cancer
Jolanta Krajewska, Aleksandra Kukulska, Malgorzata Oczko-Wojciechowska, Barbara Jarzab
Expert Review of Precision Medicine and Drug Development.2021; 6(5): 307. CrossRef - Functional evaluation of vandetanib metabolism by CYP3A4 variants and potential drug interactions in vitro
Mingming Han, Xiaodan Zhang, Zhize Ye, Jing Wang, Jianchang Qian, Guoxin Hu, Jianping Cai
Chemico-Biological Interactions.2021; 350: 109700. CrossRef - Nephrotoxicity in advanced thyroid cancer treated with tyrosine kinase inhibitors: An update
Alice Nervo, Francesca Retta, Alberto Ragni, Alessandro Piovesan, Alberto Mella, Luigi Biancone, Marco Manganaro, Marco Gallo, Emanuela Arvat
Critical Reviews in Oncology/Hematology.2021; 168: 103533. CrossRef
- Metastatic medullary thyroid carcinoma (MTC): disease course, treatment modalities and factors predisposing for drug resistance

- Clinical Study
- The Recovery of Hypothalamic-Pituitary-Adrenal Axis Is Rapid in Subclinical Cushing Syndrome
- Hee Kyung Kim, Jee Hee Yoon, Yun Ah Jeong, Ho-Cheol Kang
- Endocrinol Metab. 2016;31(4):592-597. Published online December 20, 2016
- DOI: https://doi.org/10.3803/EnM.2016.31.4.592
- 3,666 View
- 45 Download
- 8 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background In subclinical Cushing syndrome (SC), it is assumed that glucocorticoid production is insufficient to cause a clinically recognizable syndrome. Differences in hormonal levels or recovery time of the hypothalamic-pituitary-adrenocortical (HPA) axis after adrenalectomy between patients with overt Cushing syndrome (OC) and SC remain unknown.
Methods Thirty-six patients (10 with OC and 26 with SC) with adrenal Cushing syndrome who underwent adrenalectomy from 2004 to 2014 were reviewed retrospectively. Patients were treated with glucocorticoid after adrenalectomy and were reevaluated every 1 to 6 months using a rapid adrenocorticotropic hormone (ACTH) stimulation test.
Results Levels of basal 24-hour urine free cortisol (UFC), serum cortisol after an overnight dexamethasone suppression test (DST), and serum cortisol and 24-hour UFC after low-dose DST and high-dose DST were all significantly lower in patients with SC compared with OC. Basal ACTH levels showed significantly higher in patients with SC compared with OC. The probability of recovering adrenal function during follow-up differed significantly between patients with OC and SC (
P =0.001), with significant correlations with the degree of preoperative cortisol excess. Patients with OC required a longer duration of glucocorticoid replacement to recover a normal ACTH stimulation test compared with patients with SC (median 17.0 months vs. 4.0 months,P <0.001).Conclusion The HPA axis recovery time after adrenalectomy in patients with SC is rapid and is dependent on the degree of cortisol excess. More precise definition of SC is necessary to achieve a better management of patients and to avoid the risk of under- or over-treatment of SC patients.
-
Citations
Citations to this article as recorded by- Hypothalamic–pituitary–adrenal axis recovery after treatment of Cushing's syndrome
Annemarie Balasko, Karin Zibar Tomsic, Darko Kastelan, Tina Dusek
Journal of Neuroendocrinology.2022;[Epub] CrossRef - Early assessment of postoperative adrenal function is necessary after adrenalectomy for mild autonomous cortisol secretion
Trenton Foster, Irina Bancos, Travis McKenzie, Benzon Dy, Geoffrey Thompson, Melanie Lyden
Surgery.2021; 169(1): 150. CrossRef - Is Prophylactic Steroid Treatment Mandatory for Subclinical Cushing Syndrome After Unilateral Laparoscopic Adrenalectomy?
Dong Wang, Han-zhong Li, Yu-shi Zhang, Liang Wang, Zhi-gang Ji
Surgical Laparoscopy, Endoscopy & Percutaneous Techniques.2019; 29(1): 31. CrossRef - When to Intervene for Subclinical Cushing's Syndrome
Lily B. Hsieh, Erin Mackinney, Tracy S. Wang
Surgical Clinics of North America.2019; 99(4): 747. CrossRef - Serum Cortisol Levels via Radioimmunoassay vs Liquid Chromatography Mass Spectrophotometry in Healthy Control Subjects and Patients With Adrenal Incidentalomas
Martha K P Huayllas, Brian C Netzel, Ravinder J Singh, Claudio E Kater
Laboratory Medicine.2018;[Epub] CrossRef - Contralateral adrenal width predicts the duration of prolonged post‐surgical steroid replacement for subclinical Cushing syndrome
Masahiro Sugiura, Yusuke Imamura, Koji Kawamura, Satoshi Yamamoto, Tomokazu Sazuka, Kazuyoshi Nakamura, Shinichi Sakamoto, Hidekazu Nagano, Hisashi Koide, Tomoaki Tanaka, Takashi Imamoto, Akira Komiya, Tomohiko Ichikawa
International Journal of Urology.2018; 25(6): 583. CrossRef - Predictability of hypoadrenalism occurrence and duration after adrenalectomy for ACTH-independent hypercortisolism
V. Morelli, L. Minelli, C. Eller-Vainicher, S. Palmieri, E. Cairoli, A. Spada, M. Arosio, I. Chiodini
Journal of Endocrinological Investigation.2018; 41(4): 485. CrossRef - Articles inEndocrinology and Metabolismin 2016
Won-Young Lee
Endocrinology and Metabolism.2017; 32(1): 62. CrossRef
- Hypothalamic–pituitary–adrenal axis recovery after treatment of Cushing's syndrome

- Clinical Study
- Characteristics of Korean Patients with Antithyroid Drug-Induced Agranulocytosis: A Multicenter Study in Korea
- Hee Kyung Kim, Jee Hee Yoon, Min Ji Jeon, Tae Yong Kim, Young Kee Shong, Min Jin Lee, Bo Hyun Kim, In Joo Kim, Ji Young Joung, Sun Wook Kim, Jae Hoon Chung, Ho-Cheol Kang
- Endocrinol Metab. 2015;30(4):475-480. Published online December 31, 2015
- DOI: https://doi.org/10.3803/EnM.2015.30.4.475
- 4,214 View
- 58 Download
- 16 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Antithyroid drugs (ATDs) can lead to the development of agranulocytosis, which is the most serious adverse effect. Characteristics of ATD-induced agranulocytosis (AIA) have seldom been reported due to the rarity. In this study, we characterized the clinical features for AIA in Korean patients.
Methods We retrospectively reviewed data from patients with AIA diagnosed between 1997 and 2014 at four tertiary hospitals. Agranulocytosis was defined as an absolute neutrophil count (ANC) below 500/mm3.
Results The mean age of the patients (11 males, 43 females) was 38.2±14.9 years. Forty-eight patients (88.9%) with AIA had fever and sore throat on initial presentation, 20.4% of patients developed AIA during the second course of treatment, and 75.9% of patients suffered AIA within 3 months after initiation of ATD. The patients taking methimazole (
n =39) showed lower levels of ANC and more frequent use of granulocyte-macrophage colony-stimulating factor than propylthiouracil (n =15) users. The median duration of agranulocytosis was 5.5 days (range, 1 to 20). No differences were observed between the long (≥6 days) and short recovery time (≤5 days) groups in terms of age, gender, ATDs, duration of ATDs, or initial ANC levels. Four patients (7.4%) who were taking ATDs for less than 2 months died of sepsis on the first or second day of hospitalization.Conclusion The majority of AIA incidents occur in the early treatment period. Considering the high fatality rate of AIA, an early aggressive therapeutic approach is critical and patients should be well informed regarding the warning symptoms of the disease.
-
Citations
Citations to this article as recorded by- Novel Association of KLRC4-KLRK1 Gene Polymorphisms with
Susceptibility and Progression of Antithyroid Drug-Induced
Agranulocytosis
Yayi He, Pan Ma, Yuanlin Luo, Xiaojuan Gong, Jiayang Gao, Yuxin Sun, Pu Chen, Suliang Zhang, Yuxin Tian, Bingyin Shi, Bao Zhang
Experimental and Clinical Endocrinology & Diabetes.2024; 132(01): 17. CrossRef - A Disproportionality Analysis of the Adverse Effect Profiles of Methimazole and Propylthiouracil in Patients with Hyperthyroidism Using the Japanese Adverse Drug Event Report Database
Masanori Arai, Takahiro Tsuno, Hiromi Konishi, Kuniyuki Nishiyama, Yasuo Terauchi, Ryota Inoue, Jun Shirakawa
Thyroid®.2023; 33(7): 804. CrossRef - The Current Status of Hyperthyroidism in Korea
Hyemi Kwon
Endocrinology and Metabolism.2023; 38(4): 392. CrossRef - Clinical characteristics of neutropenic patients under antithyroid drug: Twelve-year experience in a medical center
Chih-Hsueh Tseng, Chi-Lung Tseng, Harn-Shen Chen, Pei-Lung Chen, Chun-Jui Huang
Journal of the Chinese Medical Association.2023; 86(9): 826. CrossRef - Association of MICA gene polymorphisms with thionamide-induced agranulocytosis
P. Ma, P. Chen, J. Gao, H. Guo, S. Li, J. Yang, J. Lai, X. Yang, B. Zhang, Y. He
Journal of Endocrinological Investigation.2021; 44(2): 363. CrossRef - Efficacy and adverse events related to the initial dose of methimazole in children and adolescents with Graves’ disease
Hyun Gyung Lee, Eun Mi Yang, Chan Jong Kim
Annals of Pediatric Endocrinology & Metabolism.2021; 26(3): 199. CrossRef - MICA polymorphisms associated with antithyroid drug‐induced agranulocytosis in the Chinese Han population
Xiaojuan Gong, Pu Chen, Pan Ma, Jiayang Gao, Jingsi Yang, Hui Guo, Chunxia Yan, Bao Zhang, Yayi He
Immunity, Inflammation and Disease.2020; 8(4): 695. CrossRef - The Management of Thyroid Disease in COVID-19 Pandemic
Won Sang Yoo, Hyun-Kyung Chung
International Journal of Thyroidology.2020; 13(2): 65. CrossRef - Increased Risk of Antithyroid Drug Agranulocytosis Associated with Amiodarone-Induced Thyrotoxicosis: A Population-Based Cohort Study
Michal Gershinsky, Walid Saliba, Idit Lavi, Chen Shapira, Naomi Gronich
Thyroid.2019; 29(2): 193. CrossRef - A Case of Acute Supraglottitis Following Anti-Thyroid Drug-Induced Agranulocytosis
Jung Jun Lee, Dong Young Kim, Jeon Yeob Jang
Journal of The Korean Society of Laryngology, Phoniatrics and Logopedics.2019; 30(2): 128. CrossRef - Association of HLA-B∗38:02 with Antithyroid Drug-Induced Agranulocytosis in Kinh Vietnamese Patients
Mai Phuong Thao, Pham Vo Anh Tuan, Le Gia Hoang Linh, Lam Van Hoang, Phan Huu Hen, Le Tuyet Hoa, Hoang Anh Vu, Do Duc Minh
International Journal of Endocrinology.2018; 2018: 1. CrossRef - Severe Gingival Ulceration and Necrosis Caused by an Antithyroid Drug: One Case Report and Proposed Clinical Approach
Ying‐Ying Chang, Chih‐Wen Tseng, Kuo Yuan
Clinical Advances in Periodontics.2018; 8(1): 11. CrossRef - Emphasis on the early diagnosis of antithyroid drug-induced agranulocytosis: retrospective analysis over 16 years at one Chinese center
Y. He, J. Li, J. Zheng, Z. Khan, W. Qiang, F. Gao, Y. Zhao, B. Shi
Journal of Endocrinological Investigation.2017; 40(7): 733. CrossRef - Association of HLA-B and HLA-DRB1 polymorphisms with antithyroid drug-induced agranulocytosis in a Han population from northern China
Yayi He, Jie Zheng, Qian Zhang, Peng Hou, Feng Zhu, Jian Yang, Wenhao Li, Pu Chen, Shu Liu, Bao Zhang, Bingyin Shi
Scientific Reports.2017;[Epub] CrossRef - Use of granulocyte colony‐stimulating factor in the treatment of methimazole‐induced agranulocytosis: a case report
Asha Birmingham, Carissa Mancuso, Craig Williams
Clinical Case Reports.2017; 5(10): 1701. CrossRef - 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis
Douglas S. Ross, Henry B. Burch, David S. Cooper, M. Carol Greenlee, Peter Laurberg, Ana Luiza Maia, Scott A. Rivkees, Mary Samuels, Julie Ann Sosa, Marius N. Stan, Martin A. Walter
Thyroid.2016; 26(10): 1343. CrossRef
- Novel Association of KLRC4-KLRK1 Gene Polymorphisms with
Susceptibility and Progression of Antithyroid Drug-Induced
Agranulocytosis


 KES
KES

 First
First Prev
Prev



